Pipeline
FDA completed safety review of our ARN-3261 IND and approved for Phase 1 1/1b clinical trials. ARN-3261 (now called GRN300) licensed and clinical Phase 1a/1b trial initiated in Q2 2021.
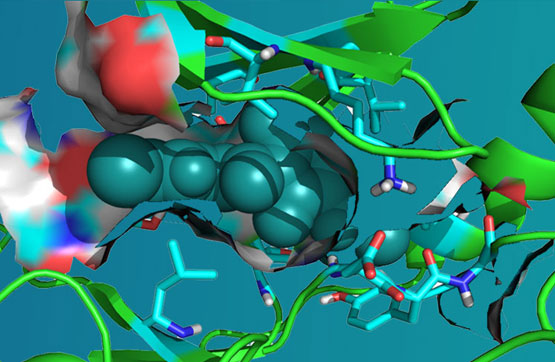
ARN-3261, is a first-in-class, orally bioavailable small molecule inhibitor of the Salt Inducible Kinases 2 and 3 (SIK2, SIK3). Three isoforms of SIK family (SIKs) protein have been reported; SIK1 (SNF1LK), SIK2 (SNF1LK, QIK) and SIK3 (QSK) are Ser/Thr centrosome kinase family members required for bipolar mitotic spindle formation.
The fact that the SIK2 kinase is overexpressed in 30% of ovarian cancer specimens underlines the clinical importance of treating ovarian cancer by blocking SIK2 kinase activity. In addition to a role in ovarian cancer, SIK2 and SIK3 are prevalent in several other tumor types including; triple-negative breast cancer, prostate cancer, diffuse large B-cell lymphoma, melanoma and AML. Inhibition of SIK2 has been reported to cause SIK2-dependent centrosome splitting in interphase, while SIK2 depletion blocked centrosome separation in mitosis, and sensitized ovarian cancers to paclitaxel in culture and in vivo xenograft models. Depletion of SIK2 also delayed G1/S transition and reduced AKT phosphorylation. Higher levels of expression of SIK2 have been shown to be significantly correlated with poor survival in patients with high-grade serous ovarian cancers. Knockdown of SIK2 with siRNA has inhibited ovarian cancer cell growth, although this methodology faces the challenges of delivering siRNA to cancer cells in vivo, underlining the potential importance of a small molecule inhibitor.
ARN-3261 inhibited ovarian tumor growth significantly in ovarian cancer animal models non-clinically. Moreover, ARN-3261 has exhibited excellent in vivo pharmacokinetic, pharmacodynamics, and correlative PK/PD and ADME characteristics. Preliminary in vitro and in vivo tumor up-take studies suggest that ARN-3261 blocks centrosome separation by inhibiting SIK2, thereby enhancing the sensitivity of paclitaxel. In addition, the clinical agent ARN-3261 exhibited promising efficacy in vivo in a dose dependent manner at orally. ARN-3261 demonstrate its activity as a single agent for treatment of ovarian carcinoma and in combination with paclitaxel, platinum and approved PARP inhibitors.