Pipeline
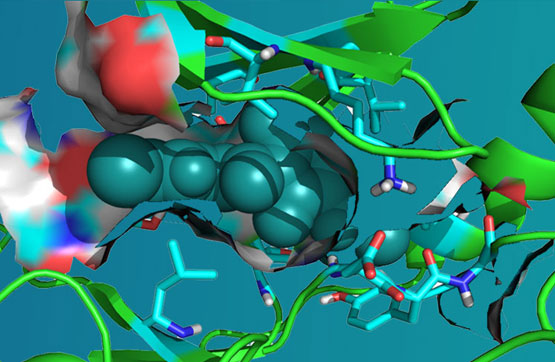
ARN-1104 is an IND ready next-generation, selective Leucine-rich Repeat Kinase 2 (LRRK2) inhibitor capable of addressing potential mechanisms that directly enhances kinase activity causing Parkinson’s Disease (PD).
The G2019S mutation in the LRRK2 ATP site enhances kinases activity that drives neurotoxicity. ARN-1104 selectively inhibits G2019S mutation in LRRK2 induced in vitro and muse model experiments slows the progression of PD symptoms.
ARN-1104 is an orally available, brain penetrant, neuroprotective agent proven to be highly tolerable and safe from the seven-day toxicology studies. ARN-1104 is an IND ready candidate and at this stage of development, company is potentially seeking an opportunity to license or partner in order to complete pending IND enabling studies to take in to clinical Phase 1 trials.